Middle School Science: Always Engaging our Students!
Middle School Science: Always Engaging our Students!
Over the past two weeks, the 7th grade science classes have been learning about acids and bases. Students studied the effect of acid rain in metals, such as copper, by placing a penny in one of the most common household acids, acetic acid, or vinegar, to all non-scientific readers. During the lab, students saw first-hand how acids can dissolve the dirt from an old penny exposing the shiny copper but then they saw how exposing it to air created a chemical reaction called oxidation, turning it green. We extrapolated these findings to explain how the Statue of Liberty (which originally was shiny brown because it's made of copper) turned green in just 30 years due to acid rain exposure. Students also studied how pollution in the air can impact not only living creatures, but man-made structures, and how looking for renewable sources of energy rather than fossil fuels could decrease the anthropogenic (man-made) effects of acid rain.
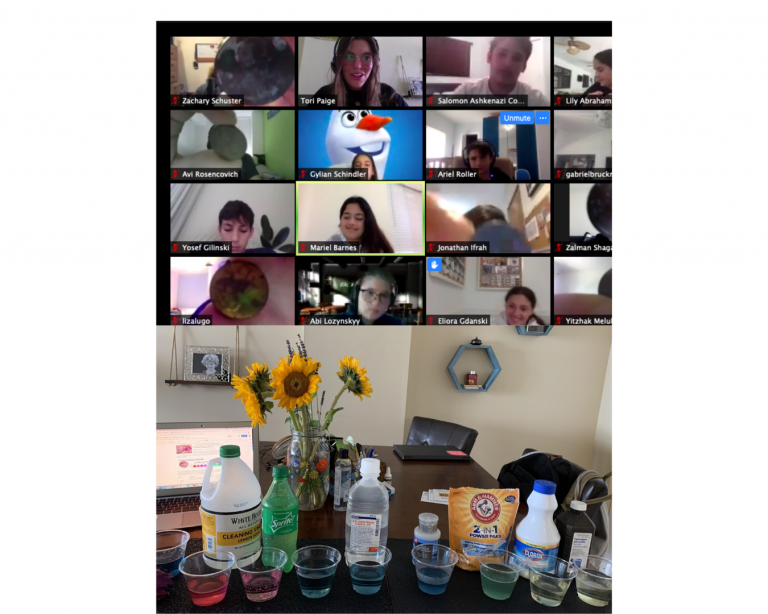
Students are learning about Acidic and Basic (alkaline) solutions by using a natural organic indicator, red cabbage. By boiling the cabbage leaves we can remove the purple pigment from the cabbage known as Anthocyanin. The liquid that is obtained will be bluish/purple and will have the property of changing colors when mixed with different substances that can range from different shades red to blue, green and yellow. Students observed Ms. Paige and Mr. Gonzalez mix the indicator with many different solutions that can be found at home. They used their observances to determine if the solution was acidic, basic or neutral.
Wishing everyone a Chag Samaech from the Middle School Team!

